Innovating with Novel Genetic Engineering Platforms
Poseida Therapeutics is advancing differentiated allogeneic cell therapies and genetic medicines by leveraging our proprietary genetic engineering platforms. Poseida was acquired by Roche in early 2025.
We utilize our novel gene insertion and gene editing technologies to introduce or edit genes, which may enable our investigational therapies to treat a range of cancers, autoimmune, and rare diseases. Our proprietary, differentiated tools lay the foundation for potentially safer and more effective treatments.
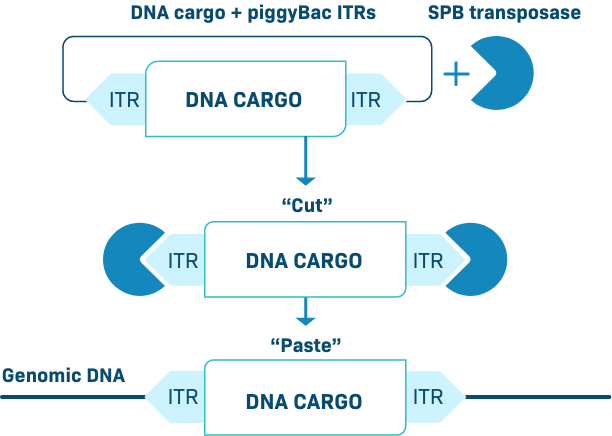
INSERTION PiggyBac Platform
The piggyBac transposon is a cut-and-paste gene insertion platform that can stably integrate DNA into the genome. This is enabled by a high efficiency transposase which accommodates a large DNA cargo. We have developed an optimized piggyBac transposase as an enhanced proprietary system we call Super piggyBac.
Our piggyBac DNA delivery system efficiently delivers genetic cargo into both resting and activated/dividing cells. During the CAR-T manufacture process, it preferentially modifies naïve T cells and stem cell memory T cells (Tscm), thereby creating products enriched for an early memory phenotype.
Our data suggest that piggyBac is ideal for introducing therapeutic DNA transgenes to growing tissues, such as the juvenile liver. Therapies created with piggyBac offer a potentially more consistent and durable treatment response.
PiggyBac can deliver genetic payloads to a wide variety of cell types (in vivo or ex vivo), including immune cells and hepatocytes, resulting in durable transgene expression. As demonstrated in T cells, piggyBac can easily carry multiple targeting genes like CARs and/or a TCR, a gene encoding a safety switch, and a selection gene – all without the need for viral-based transgene delivery.

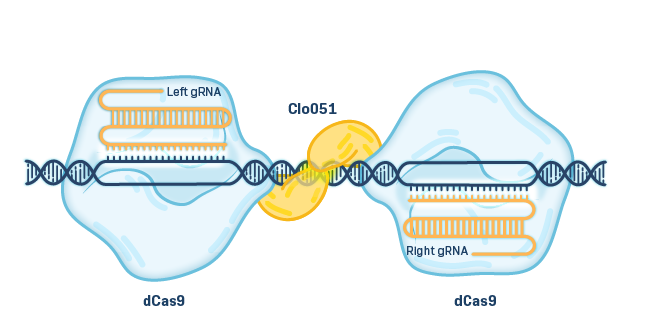
EDITING Cas-CLOVER Platform
Cas-CLOVER is a high-fidelity, proprietary gene editing technology with low to no off-target activity. Cas-CLOVER utilizes a deactivated Cas9 (dCas9) that acts as a binding protein when combined with a guide RNA. Rather than cutting the DNA, the dCas9 guides a fused nuclease domain from the Clo051 enzyme (CLOVER), which can only cut DNA when bound to its matching pair, giving it enhanced fidelity via a highly specific molecular address.
Cas-CLOVER is effective in resting T cells, which helps preserve an early T cell memory phenotype, and is capable of making multiple gene edits in the same cells. Advantages of Cas-CLOVER include ease of design in cell therapy, lower cost, and multiplexing capabilities.

DELIVERY Leading Gene Delivery Systems
We have multiple gene delivery technology platforms, including our fully non-viral nanoparticles, which can deliver large genetic material to multiple cell types in vivo or ex vivo.
We aim to match the right tools and cargo with the right delivery mechanism for each indication to achieve long-term, durable gene expression – with the goal of developing effective, potentially functional cures.

BENEFITS Additional Benefits in Cell Therapy
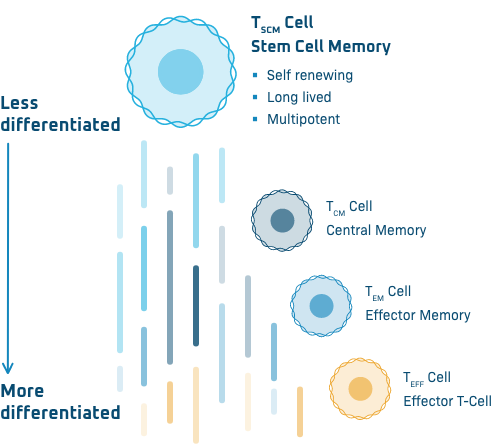
TSCM Cells
At Poseida, our allogeneic, or off-the-shelf, CAR-T products are produced with a high percentage of desirable TSCM cells, or stem cell memory T cells. These cells are long-lived, self-renewing, and capable of differentiating into the entire spectrum of T cell subsets primed to lead the effector response against the cancer. Data have shown that TSCM cells can survive for years after CAR-T treatment, potentially providing durable anti-tumor protection.
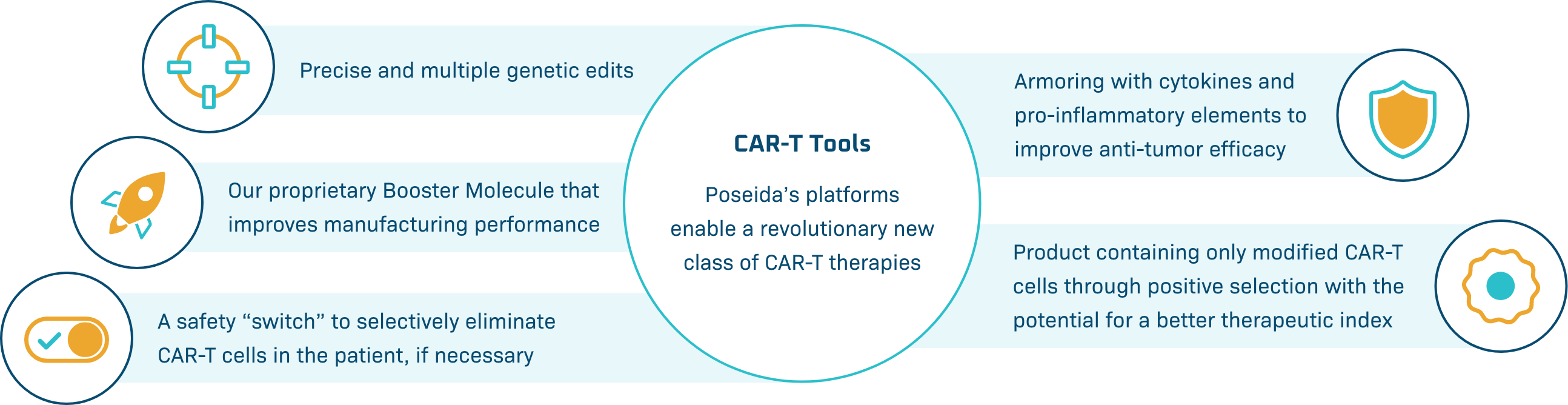
CAR-T Tools
Poseida’s platforms enable a revolutionary new class of CAR-T therapies
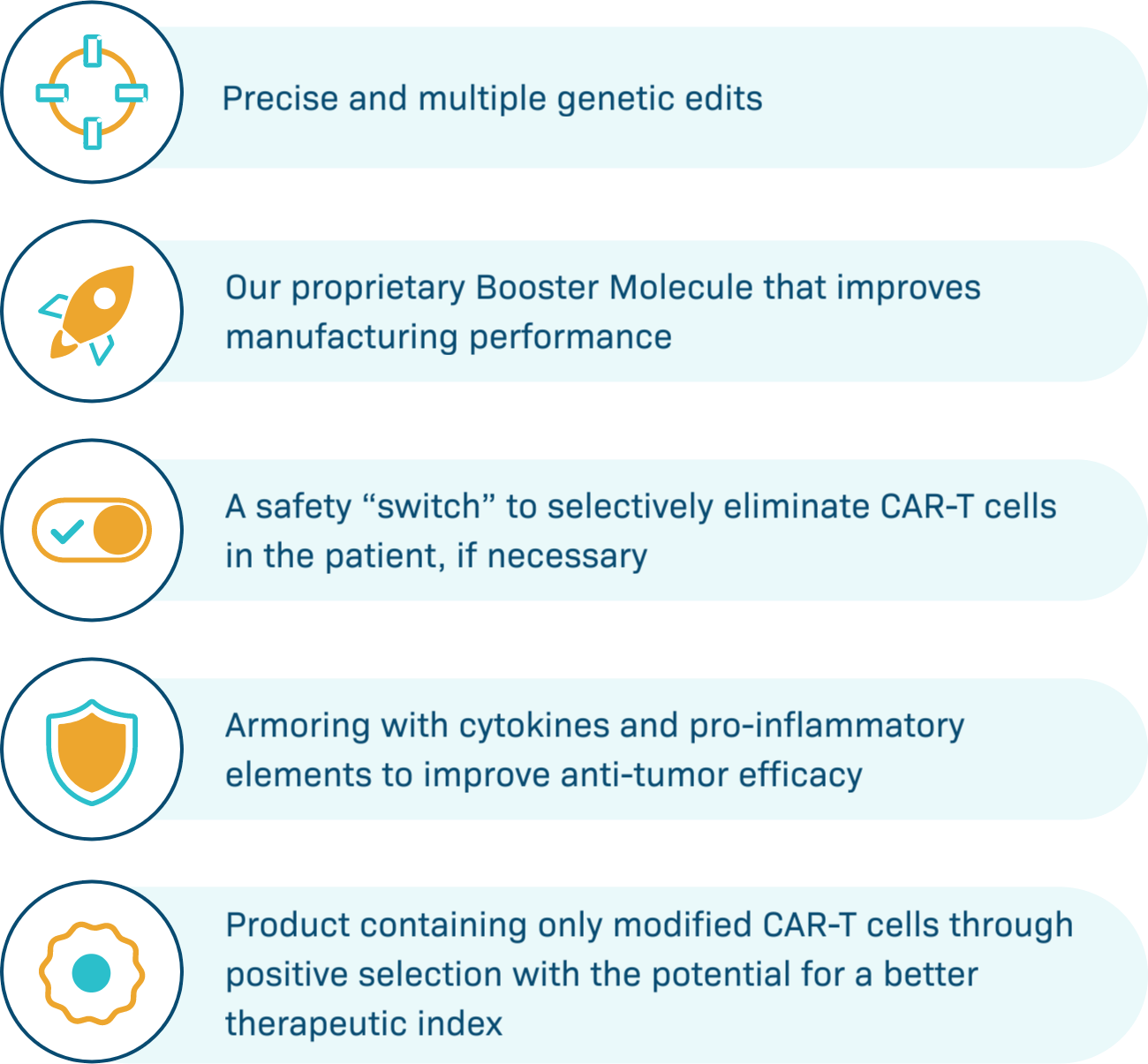
Unique to Poseida
Our platforms offer large cargo capacity and precise gene editing, with the potential for stable, long-term gene expression. Engineered to provide potentially better tolerability, maximal potency, targeted delivery, and expression in diverse cell types, we believe Poseida’s therapeutic candidates offer the capacity to cure cancer and genetic diseases.
